Electrochemical Sensors
graduate students: Maher, Ekram, Rasha, Shereen, Sayed and Hany
One of the major research directions in our lab is electrochemical sensors based on conducting polymers (CPs). Our research group has been leading in this area since its beginning in the late 1980s. Conducting polymers have attracted our interest in that they exhibit electronic, magnetic and optical properties of metals and semiconductors while retaining the attractive mechanical properties and processing advantages of polymers. These features, along with chemical sensitivity, room temperature operation, and tunable charge transport properties, have positioned conducting polymers as a major class of chemical transducers, creating powerful thin/thick film sensors for over two decades. However, there is always the desire to further optimize conducting polymers when targeting a specific application. We have developed many approaches in our lab for increasing the sensitivity/selectivity of conducting polymers to analytes by the incorporation of synthetic or natural receptors. One such approach is the incorporation of metal nanoparticles into the conducting polymers. The porous structure of conducting polymer allows dispersing the metal nanoparticles into the polymer matrix and generates additional electrocatalytic sites (Figure 1). This represents one of the most recent approaches for the sensitive and selective determination of organic compounds and those of biological and biochemical interest (Figure 2) and produces among the lowest electrochemical limit of detection reported to date [1-5].
Another approach consists of the introduction of synthetic receptors such as cyclodextrines [6], ferrocenes [6], crown ethers [7] and surfactants [8-11] which improve the sensitivity of conducting polymers to ions, organic molecules and gases. Of increasing interest, our lab have explored and developed ideas on using surfactants as secondary components with conducting polymer for the selective and sensitive determination of compounds of biological and pharmaceutical interest [8-11]. Additionally, sensors based on self-assembly at nanoparticles-modified electrodes (Figure 3) are currently under investigation in our lab.
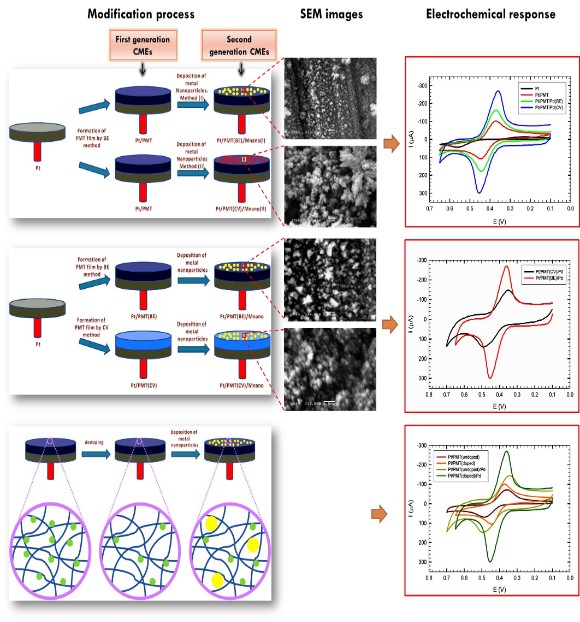
Figure 1: Conducting polymer/metal nanoparticles composites form the basis of highly selective and sensitive sensors; (a) Schematic showing two-step process for the preparation of conducting polymer/metal nanoparticle-modified electrode. In the first step, a polymer film is formed on the electrode (first generation CMEs) (see fig. 3 for the various types of CMEs). In the second step, metal nanoparticles are dispersed to the polymer matrix (second generation CMEs). Obviously, different structures, morphologies and electrochemical responses result when using different synthesis conditions [2].
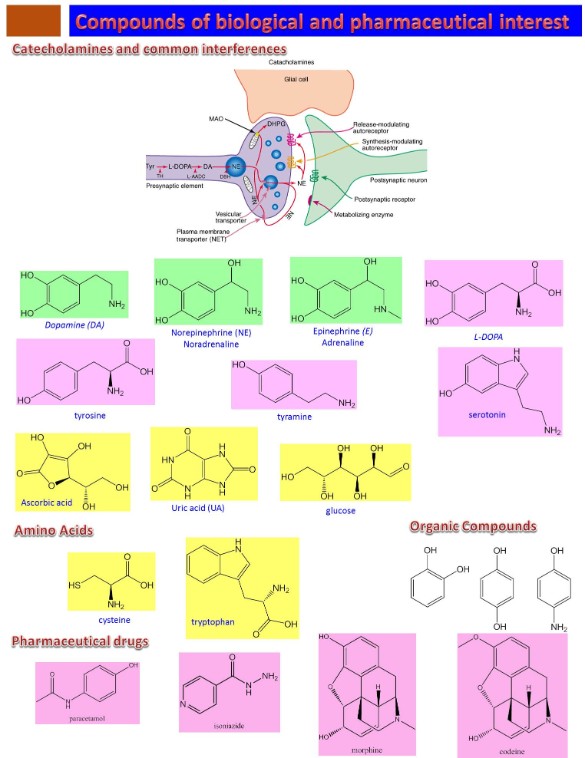
Figure 2: catecholamines, amino acids and some pharmaceutical drugs have found interest in our lab as sensing targets.
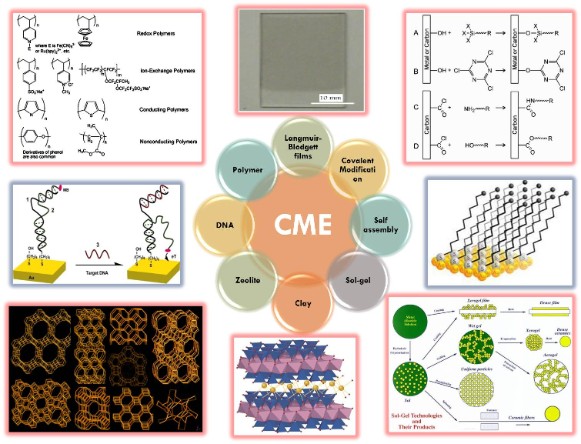
Figure 3: various types of chemically modified electrodes (CMEs).
Selected Publications:
1- "Chemical Sensors and Biosensors based on conducting polymer thin films", Nada F. Atta, Ahmed Galal and Maher F. El-Kady. A book chapter in an edited book “Polymer Films: Properties, Performance and Applications” by NOVA publishers, New York, USA. The book will be published in the 2nd quarter of 2011. See the following link at the publisher’s webpage https://www.novapublishers.com/catalog/product_info.php?products_id=16388.
2- Novel poly(3-methylthiophene)/Pd, Pt nanoparticle sensor: Synthesis, characterization and its application to the simultaneous analysis of dopamine and ascorbic acid in biological fluids, Nada F. Atta, Maher F. El-Kady, Sens. Actuators, B. 141, 566-574, (2010). Full Article
3- Simultaneous determination of catecholamines, uric acid and ascorbic acid at physiological levels using poly(N-methylpyrrole)/Pd-nanoclusters sensor, Nada F. Atta, Maher F. El-Kady and Ahmed Galal, Anal. Biochem., 400, 78-88, (2010). Full Article
4- Palladium nanoclusters-coated poly(furan) as a novel sensor for catecholamine neurotransmitters and paracetamol, Nada F. Atta, Maher F. El-Kady, A. Galal, Sens. Actuators, B, 141, 566-574, (2009). Full Article
5- Carbon Paste Gold Nanoparticles Sensor for the Selective Determination of Dopamine in Buffered Solutions, Nada F. Atta, Ahmed Galal, Fekria M. Abu-Attia, and Shereen M. Azab, J. Electrochem. Soc., 157(9), F116-F123, (2010). Full Article
6- Physico-chemical studies on some electrochemically prepared organic polymers, Hany Issa, Master’s Thesis, Chemistry Department, Faculty of Science, Cairo University (2008).
7- Poly(Binaphthyl-20-Crown-6) as Receptor Based Molecular Selective Potentiometric Electrodes for Catecholamines and 1,2-Dihydroxybenzene Derivatives, Y. Long Ma, Ahmed Galal, S. K. Lunsford, H. Zimmer, H. B. Mark, Jr., Z. F. Huang and P. B. Bishop, Biosensors and Bioelectronics, 10, 705-15 (1995). Full Article
8- Effect of surfactants on the voltammetric response of an antihypertensive drug, Nada F. Atta, Soher A. Darwish, Sayed S. Khalil, A. Galal, Talanta, 72, 1438-1445, (2007). Full Article
9- Poly(3,4-ethylene-dioxythiophene) electrode for the selective determination of dopamine in presence of sodium dodecyl sulfate, Nada F. Atta, Ahmed Galal, Rasha A. Ahmed, Bioelectrochemistry, 80, 132-141 (2011). Full Article
10- Characterization and electrochemical investigations of micellar/drug interactions, Nada F. Atta, Ahmed Galal, Fekria M. Abu-Attia, Shereen M. Azab, Electrochim. Acta, 56, 2510-2517 (2011). Full Article
11- Direct and simple electrochemical determination of morphine at PEDOT modified Pt electrode, Nada F. Atta, Ahmed Galal, Rasha A. Ahmed, Electroanalysis, 23, 737-746, (2011). Full Article